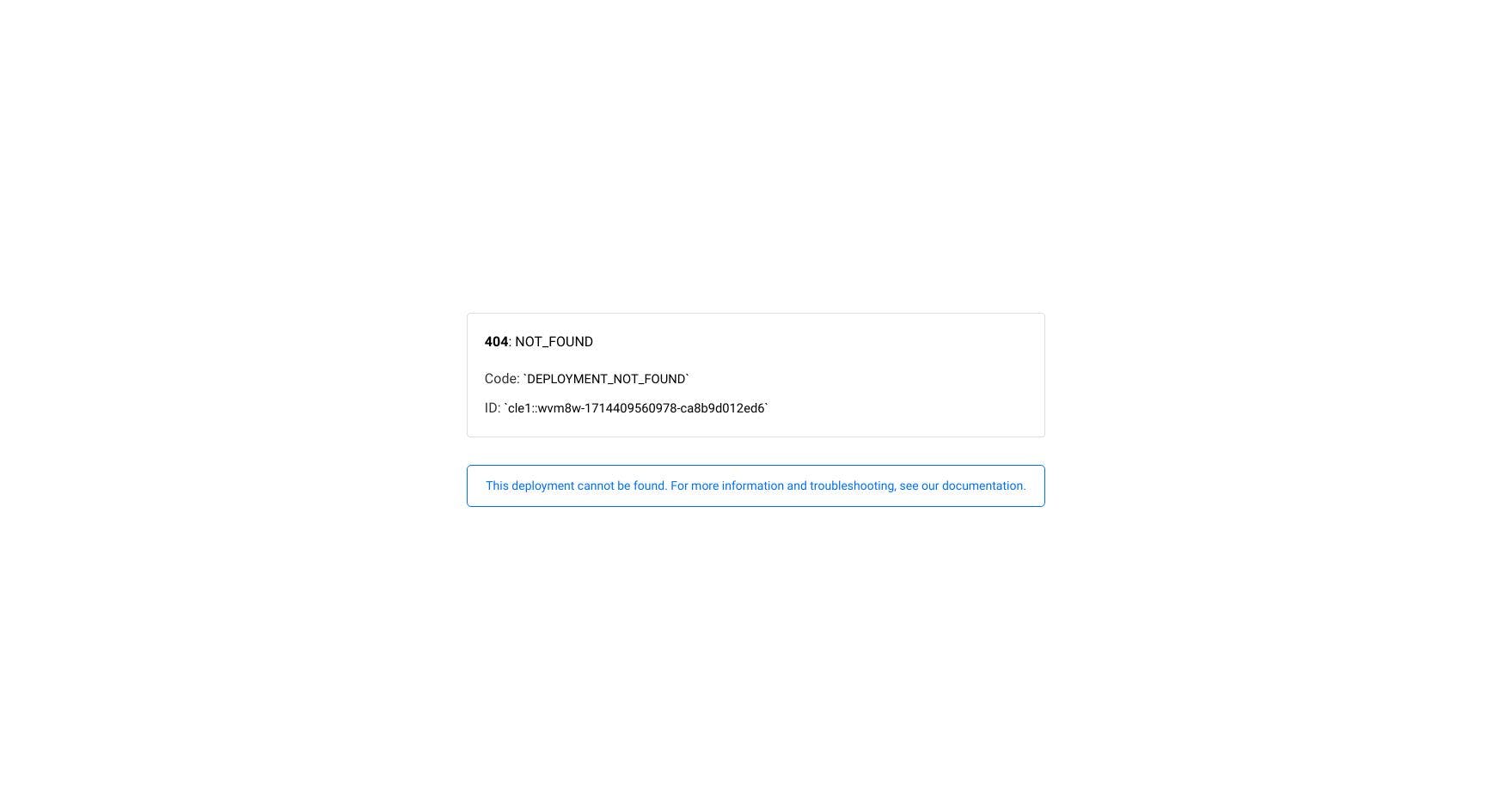
DTIL VS PASG Stock Comparison
Performance
DTIL10/100
10/100
DTIL returned -79.21% in the last 12 months. Based on SPY's performance of -20.66%, its performance is below average giving it a score of 10 of 100.
PASG10/100
10/100
PASG returned -57.31% in the last 12 months. Based on SPY's performance of 13.09%, its performance is below average giving it a score of 10 of 100.
Sentiment
DTIL68/100
68/100
DTIL had a bullish sentiment score of 68.05% across Twitter and StockTwits over the last 12 months. It had an average of 8.75 posts, 4.76 comments, and 13.29 likes per day.
PASG73/100
73/100
PASG had a bullish sentiment score of 73.36% across Twitter and StockTwits over the last 12 months. It had an average of 6.76 posts, 1.48 comments, and 2.95 likes per day.
Technicals
DTIL14/100
14/100
DTIL receives a 14 of 100 based on 14 indicators. 2 are bullish, 12 are bearish.
PASG10/100
10/100
PASG receives a 10 of 100 based on 14 indicators. 0 are bullish, 12 are bearish.
Earnings
DTIL10/100
10/100
DTIL has missed earnings 5 times in the last 20 quarters.
PASG10/100
10/100
PASG has missed earnings 8 times in the last 20 quarters.
Profit
DTIL10/100
10/100
Out of the last 20 quarters, DTIL has had 1 profitable quarters and has increased their profits year over year on 1 of them.
PASG10/100
10/100
Out of the last 19 quarters, PASG has had 0 profitable quarters and has increased their profits year over year on 1 of them.
Volatility
DTIL32/100
32/100
DTIL has had a lower than average amount of volatility over the last 12 months giving it a score of 32 of 100.
PASG35/100
35/100
PASG has had a lower than average amount of volatility over the last 12 months giving it a score of 35 of 100.
All score calculations are broken down here to help you make more informed investing decisions
Precision BioSciences, Inc. Common Stock Summary
Nasdaq / DTIL
Healthcare
Biotechnology
Precision BioSciences, Inc., a clinical stage gene editing company, develops in vivo gene editing and ex vivo allogeneic CAR T therapies in the United States. It offers ARCUS, a genome editing platform to cure genetic disorders. The company also provides Ex vivo Allogeneic CAR T Immunotherapy, a form of immunotherapy in which T cell, a specific type of immune cell is genetically engineered to recognize and kill cancer cells; PBCAR0191, which is in Phase 1/2a clinical trial in adult patients with R/R NHL or R/R B-cell precursor acute lymphoblastic leukemia, or B-ALL; PBCAR19B, an anti-CD19 CAR T candidate built on the stealth cell platform utilizing a single-step gene edit to minimize the risk of chromosome abnormalities; and PBCAR269A, an investigational allogeneic CAR T immunotherapy targeting BCMA for the treatment of R/R multiple myeloma. The company has development and commercial license agreement with Les Laboratoires Servier to develop allogeneic chimeric antigen receptor T cell therapies for antigen targets, hematological cancer targets beyond CD19, and solid tumor targets; Tiziana Life Sciences to evaluate foralumab, a fully human anti-CD3 monoclonal antibody as a lymphodepleting agent for the potential treatment of cancers; and iECURE, Inc. to develop ARCUS-based gene editing therapies. Precision BioSciences, Inc. was incorporated in 2006 and is headquartered in Durham, North Carolina.
Passage Bio, Inc. Common Stock Summary
Nasdaq / PASG
Healthcare
Biotechnology
Passage Bio, Inc., a genetic medicines company, develops transformative therapies for central nervous system diseases. It develops PBGM01, which utilizes a proprietary, AAVhu68 capsid to deliver to the brain and peripheral tissues a functional GLB1 gene encoding lysosomal acid beta-galactosidase for infantile GM1; PBFT02, which utilizes an AAV1 capsid to deliver to the brain a functional granulin (GRN) and gene encoding progranulin (PGRN) for the treatment of FTD-GRN; and PBKR03, which utilizes a proprietary, AAVhu68 capsid to deliver to the brain and peripheral tissues a functional GALC gene encoding the hydrolytic enzyme galactosylceramidase for infantile Krabbe disease. The company also develops PBML04 for the treatment of metachromatic leukodystrophy; PBAL05 for the treatment of amyotrophic lateral sclerosis; and PBCM06 for the treatment of Charcot-Marie-Tooth Type 2A. Passage Bio, Inc. has a strategic research collaboration with the Trustees of the University of Pennsylvania's Gene Therapy Program; and collaboration agreement, and a development services and clinical supply agreement with Catalent Maryland, Inc. The company was incorporated in 2017 and is headquartered in Philadelphia, Pennsylvania.
Power up your portfolio
Build a portfolio of your favorite stocks
Pluto makes it easy to build investing strategies and automate your portfolio
Compare Similar Companies
Compare DTIL to other companies in the same or a similar industry.